Home Care Referral Considerations
Things to keep in mind:
- Homecare is meant for short-term, intermittent care of patients
- The patient’s insurance dictates what homecare can provide and must follow regulations for payment
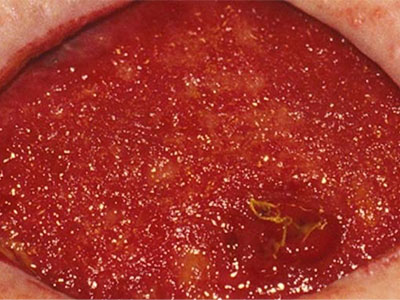
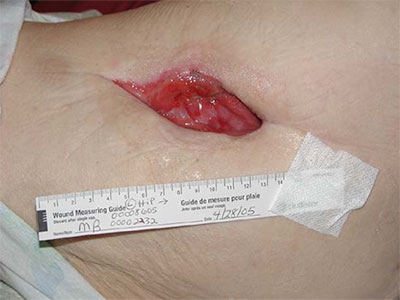
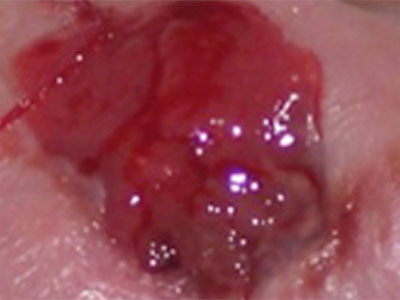
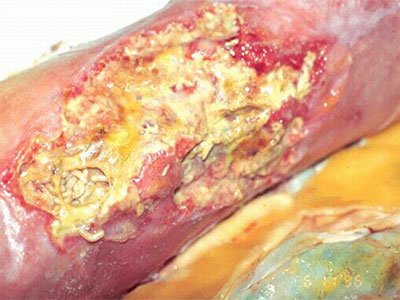
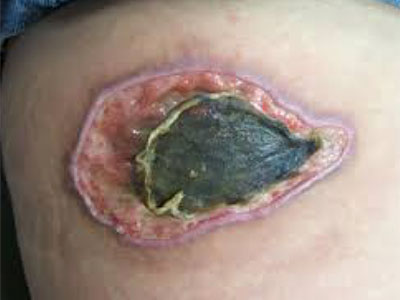
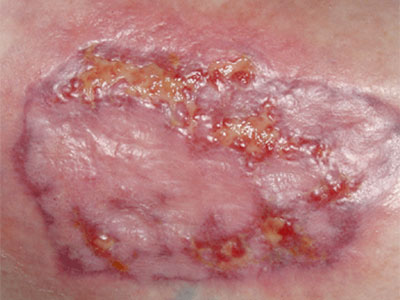
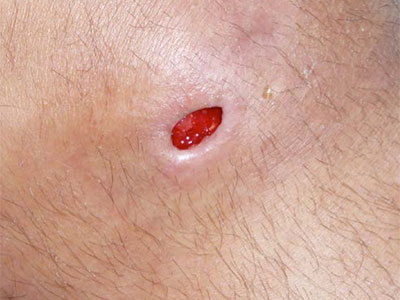
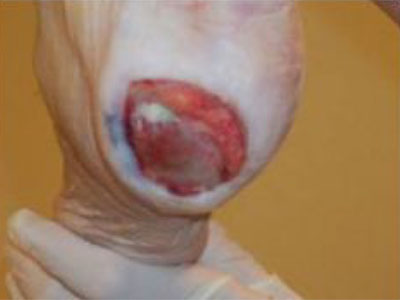
- Wound care needs to be skilled, medically necessary and medically appropriate according to evidence-based best practice needing nursing oversight
- Specific examples of non-skilled wound care include:
- Applying topical ointment
- Dry dressing only
- Wet to dry dressings
- Application of pressure wraps with no open wounds
- To provide comprehensive care, please notify any other physician active in patient care of your referral to homecare
Insurance considerations:
Medicare/Medicare HMO patient must be homebound (trouble leaving home without help [e.g., using a cane, wheelchair, walker, or crutches]; needing special transportation; or requiring help from another person) because of an illness or injury, or leaving home isn't recommended because of condition, normally unable to leave home because it's a major effort